CystagonTM is cysteamine bitartrate. Cysteamine levels clear very quickly from blood, but the response of intracellular cystine is slower. Still, after a couple of hours, the intracellular cystine begins to rise again. If Cystagon is taken at longer intervals, there will be periods with high cystine levels. There is evidence that accumulation of cystine occurs in the morning if Cystagon is not taken every 6 hours (Levtchenko EN, et al. Strict cysteamine dose regimen is required to prevent nocturnal cystine accumulation in cystinosis. Pediatr Nephrol. 2006 Jan;21(1):110-3).
2. Why is ProcysbiTM given every twelve hours?
ProcysbiTM is a microencapsulated formulation of cysteamine which has delayed release. In general, compared to unencapsulated cysteamine, blood levels appear later with a lower peak, and levels are detectable for longer. Patients have been able to attain satisfactory intracellular cystine levels 12 hours after a dose (Langman CB, et al. A randomized controlled crossover trial with delayed-release cysteamine bitartrate in nephropathic cystinosis: effectiveness on white blood cell cystine levels and comparison of safety. Clin J Am Soc Nephrol. 2012 Jul;7(7):1112-20).
3. Why is CystaranTM given so frequently?
CystaranTM is a specially formulated solution to be addded to the eye to prevent crstal formation in the cornea. Do not add other liquid cysteamine solutions to the eye. Cysteamine can be oxidized quickly, so the stability of CystaranTM is limited. The recommendation is to give it every hour while awake. See cystaran.com
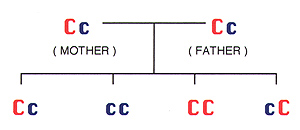
4. How is cystinosis inherited ("passed on")? What are the chances of having a cystinotic child after the first child is born?
C = normal gene / non-cystinosis gene
c = recessive gene /cystinosis gene
Cc = carrier / heterozygote / no cystinosis
CC = non carrier / no cystinosis
cc = cystinosisThis is the pattern of autosomal recessive inheritance.
Carriers have normal health. Every time that two people who are carriers for the cystinosis gene have a child, there is a:
-- 1 in 4 chance the child will have cystinosis (cc)
-- 2 in 4 chance the child will not have cystinosis, but will be a carrier (Cc, cC)
-- 1 in 4 chance the child will neither have cystinosis nor be a carrier x (CC)
-- 2 in 3 chance that an unaffected/non-manifesting child will be a carrier (Cc+ cC) / (Cc+ cC+ CC)
5. Do carriers of the cystinosis gene have any symptoms?
There are no known kidney or general health problems in carriers (heterozygotes) of cystinosis.
6. When should cysteamine be started?
Early diagnosis and treatment is critical, because approximately one year of maintenance of renal function is achieved for every month of early cysteamine therapy, i.e., treatment prior to 2 years of age. (Markello TC, et al. Improved renal function in children with cystinosis treated with cysteamine. N Engl J Med 1993;328: 1157-62).7. Can a female take CystagonTM during pregnancy?
The offical answer is "No". There are concerns about teratogenic effects (i.e., damaging effects on the structural development of a fetus) in animal studies, and altbough there are no known reports of this having happened to babies born to women who were treated while pregnant, we do not know if there are similar effects when pregnant women use cysteamine.
8. Does the dose of cysteamine need to be modified if a patient has decreased kidney function?
No. Reduced kidney function (glomerular filtration rate) does not correlate with cysteamine levels, since only a small amount of cysteamine is eliminated by the renal system.
9. Can CystagonTM be given while a patient is on dialysis?
Yes. Cysteamine may be filtered somewhat more with dialysis than with the native renal system The dose of cysteamine ned not be adjusted due to decreased renal function, but should be adjusted in order to keep white blood cell cystine levels in the therapeutic target range.
10. Will a transplanted kidney develop cystine crystals and cause renal failure?
No. Studies have shown that trasplanted kidneys in non-cystinosis patients. In addition, the cystine crystals found in transplanted kidneys of cystinosis patients are in the host cells, which have migrated into the donor kidneys, and are not derived from the donor kidneys themselves. (Bill Gahl)11. If cysteamine therapy is stopped for several days do I have to start back on the cysteamine slowly?
Not necessarily. There is no medical problem expected when restarting a full dose of cyteamine. But it could be a personal preference to slowly resume, if a given patient finds it difficult to reacclimate to a full dose.
12. If is possible to overdose on cysteamine? What happens if a double dose of cysteamine is given?
Yes. Acute overdose is possible, but (by anecdotal report) doses more than 6 times the recommended dose have caused vomiting but nothing else. Large overdoses may be serious, but with double dosing, observe closely, plan to skip the next dose, and re-evaluate after that. Chronic overdosage (>1.95 mg/m2/day) has been assoicated with skin lesions (bruising, with striae, identified as angioendotheliomatosis (Besouw MT, et al. Cysteamine toxicity in patients with cystinosis. J Pediatr. 2011 Dec;159(6):1004-11). There is evidence that copper depletion may play a role in that side effect (Besouw MT, et al. Copper deficiency in patients with cystinosis with cysteamine toxicity. J Pediatr. 2013 Sep;163(3):754-60), but the problem has only arisen so far with sustained high doses of cysteamine.
13. Does carnitine help patients with cystinosis? Are there any side effects of carnitine? And is the carnitine in the health food stores safe to use?
Carnitine may be lost from the body along with the other solutes not reabsorbedd by the kidney. As a result, sometimees blood carnitine levels are lowered. When plasma carnitine levels are low, oral carnitine can rapidly return them to normal. Muscle carnitine levels can also be restored with oral carnitine treatment, but there is no evidence of any beneficial effects on muscle size or strength. Side effects of carnitine include occasionaly nausea, upset stomach, or diarrhea, and a fish smell to the skin if large doses are taken. All of those are dose-dependent and reversible. There is some concern more recently with metabolite of carnitine (trimethylamine oxide, or TMAO, and gamma-butyrobetaine) which might increase risk of atherosclerosis, in older age and with chronic carnitine use (Koeth RA, et al. Intestinal microbiot metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013 May;19(5):576-85).. The carnitine in health food stores, now that D-carnitine has been eliminated, is safer than before, but I would recommend only prescription-brand carnitine. (Bill Gahl)
14. Is it safe to take other medications with cysteamine, and are there any known drug interactions?
It is safe. There appear to be no specific drug interactions.
15. What is the life expectancy of a person with cystinosis?
That has not been determined. The first gneration of patients with classical cystinosis who were treated with cysteamine early in life is now in its 4th decade, and there are individuals in their 6th decade. But as patients with early cysteamine therapy get older, they will define the life expectancy. It is reasonable to proejct a normal life span for a young patient nowadays.
16. Does cystinosis affect the immune system?
Thre is no evidence of a specific immune deficiency in cystinosis.
17. In which countries does cystinosis occur?
Cystinosis is a panethnic disease; it is found in all populations in all countries. Cystinosis is more frequent in northern Europe, and a specific mutation (the 57kb deletion) is more commonly seen in cystinosis patients from that part of the world. Cystinosis is undoubtedly under-diagnosed in many parts of the world.
18. Can a cystinotic child outgrow the need for medications such as Polycitra?
Electrolyte and mineral requirements losses, and requirements for replacement medications such as Polycitra, increase as glomerular filtration increases and solute reabsorption decreases. The requirements will vary during the individual patient's lifetime. The defect in reabsorption doesn't improve with age. Glomerular filtration decreases with age in anyone, but decreases rapidly if renal failure progresses. Dosing of replacement medications must be individualized.
19. Is is safe to give growth hormone to a child with cystinosis?
Yes. Many cystinosis children have recieved it with some benefit.
20. Do individuals with cystinosis have difficulties with swallowing? And is there anything to treat this problem?
A late complication, often encountered at advanced age, has been weakness of the "strap" muscles of the neck which affects swallowing, and this can be a serious problem. This seems to be less severe when the paient is most compliant with cysteamine treatment.
21. Do cystinotics have unusual eating habits? Has anyone conducted a nutritional study?
Young children have a strong tendency to favor salty and spicy foods. This may be at least in part due to craving for salt because of incresed mineral and electrolyte requirements.
22. Is is too late to start taking cysteamine as an adult? Are there any benefits in taking cysteeamine as an adult?
No. Aside from the benefits in terms of kidney, cysteamine helps delay and/or reduce the late, extra-renal complications, such as hypothyroidism, diabetes, vision problems, difficulty swallowing, and other muscle problems.
23. Can I regulate the amount of water my child drinks?
It is important to give as much water as the child needs, and because of renaltubular loss of water and electrolytes, the requiremens can be very large. It is dangerous to limit intake below the requirement.
24. What is the difference between cysteamine HCl, phosphocysteamine and cysteamine bitartrate?
Cysteamine HCl, the hydrochloride salt, is the form of cysteamine used in CystaranTM eye drops. Bysteamine bitartrate is another salt form which is less prone to absorb water from the air, and it is the form used in CystagonTM tablets. ProcysbiTM also contains cysteamine bitartrate, but microencapsulted in beds which do not release until they go past the stomach. Phosphocysteamine is another formulation (a phosphate ester) which was tried around the time that Cystagon was being developed. There were felt to be no practical advantages of phosphocystamine, and it was not developedd as a medication.